How the Program Works
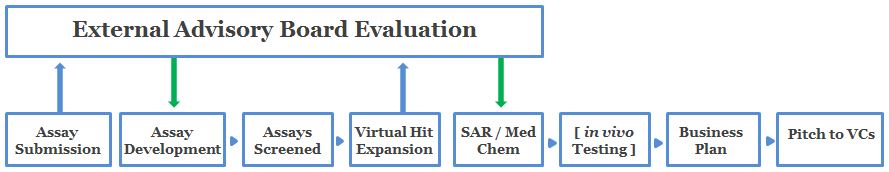
The diagram below illustrates the flow of information throughout the PITCH program. More detailed information is provided in the text below.
Please review our Frequently Asked Questions page for additional information.
Application Submission
- Eligible applicants are scientists from Yale with compelling projects that meet a new demand or offer a significant improvement over an existing treatment. Applicants need to plan to be available, either through electronic presentation (Zoom) or in person, to present their applications to the External Advisory Board on the posted presentation day for that application cycle.
- Requests for Applications is currently closed.
- Informational seminars describing the program and application will be scheduled on Yale campuses.
- The application format is non-traditional! Applicants should fill in the template PowerPoint slide deck with relevant information. Helpful Frequently Asked Questions (FAQs) and tips can be found in “Notes” view within PowerPoint.
- Please provide a few publications to provide context for and support your application. Specifically, you can submit up to two review articles that describe therapeutic area, target of your research, or connection between them, and up to two research articles that describe advances relevant to the science of your project. The publications need not be work from your laboratory. You can highlight sections to draw reviewers’ attention. Please send the articles as pdfs to pitch@yale.edu. This additional information will be tangibly useful for reviewers in evaluating the applications.
- The review committee expects completed PowerPoint slidedecks and may compare information among applications.
- The template PowerPoint Application can be found here.
- An explanation of Faculty expectations can be found here.
-
Please send applications by email to pitch@yale.edu. Application receipt will be confirmed. Check back soon for future application deadlines.
External Advisory Board (EAB) Evaluation (Round I)
- EAB will preview applications and invite applicants of top ranking proposals to present their slidedeck.
- Each applicant will have 12 minutes to present their deck and 8 minutes for additional questions. Feedback from the EAB will be in person and in real time.
- Top ranked applications will be selected for Assay Submission or the appropriate entry point for that individual project into the program.
Assay Development
- Assays will be transferred to YCMD for development, optimization and robotic testing.
- A thorough plan for any proprietary reagents to support the work will be developed.
- Updates on the progress will be provided quarterly or upon completion of this phase.
Assays Screened
- Primary screening will be performed at YCMD.
- Thresholds for identifying hits will be determined based on the distribution of primary screening data.
- Secondary testing to confirm screen active molecules will be performed which may include orthogonal assay testing, specificity testing and potency testing.
- Medicinal chemists at Yale will prioritize the best chemical matter and cluster active molecules.
Virtual Hit Expansion
- Virtual Hit Expansion will be conducted to support preliminary SAR (SAR by catalog) and generate novel lead matter.
- Data will be included in to the powerpoint application for evaluation by the EAB in Round II.
The External Advisory Board (EAB) Evaluation (Round II)
- The EAB will evaluate projects based on the quality of lead matter, compelling biology and commercial opportunity.
- Because costs associated with the next step in the program are resource-intensive, we expect significant attrition at this project milestone.
- Projects that are not initially selected for SAR/Med chem may become more competitive in subsequent evaluation rounds if significant biological validation or further development of the commercial plan are undertaken by the applicant.
SAR/Med Chem
- SAR/Med Chem will be conducted by the host institution. Iterative rounds of chemical design, synthesis and testing will be conducted to improve hit matter and generate usable lead matter.
- Lean Startup principles are to achieve a “minimal viable product” for testing and evaluation.
In Vivo Testing
- Lead molecules will be evaluated in systems relevant for their intended use.
- Some tests may be outsourced to CROs (solubility, permeability, binding), whereas others may take place at Yale (small scale animal studies).
- Business Plan
- Program data will be compiled in preparation for the VC PITCH.
- Applicants will draw on resources at Yale to refine their slidedecks and business case, and develop skills critical for successful presentation.
VC PITCH
- Venture capital firms will be contacted for presentation.
For questions about the program or application, please send to pitch@yale.edu or contact Francine Carland at francine.carland@yale.edu or 203-737-3864.